

Dupuytren's contracture or palmar fibromatosis (also known as Dupuytren's disease, morbus Dupuytren, or the slang term "Viking disease"), is a flexion contracture of the hand due to a palmar fibromatosis, in which the fingers bend towards the palm and cannot be fully extended (straightened). It is an inherited proliferative connective tissue disorder that involves the hand's palmar fascia. It is named after Baron Guillaume Dupuytren, the surgeon who described an operation to correct the affliction.
| Dupuytren's contracture | |
|---|---|

Dupuytren's contracture of the ring finger
|
Dupuytren contracture is treated with procedures to help straighten the fingers, but this does not cure the underlying disease. Contractures often return or involve other fingers.
The ring finger and little finger are the fingers most commonly affected, but any and all digits may be involved. Dupuytren contracture progresses slowly and is often accompanied by some aching and itching. In patients with this condition, the palmar fascia (palmar aponeurosis) thickens and shortens so that the tendons connected to the fingers cannot move freely. The palmar fascia becomeshyperplastic and contracts.
Incidence increases after age 40; at this age, men are affected more often than women. Beyond 80 the gender distribution is about even.
Signs and symptoms
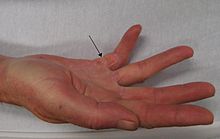
Typically, Dupuytren's contracture first presents as a thickening or nodule in the palm, which initially can be with or without pain. Later in the disease process, there is increasing painless loss of range of motion of the affected fingers. The earliest sign of a contracture is a triangular “puckering” of the skin of the palm as it passes over the flexor tendon just before the flexor crease of the finger, at themetacarpophalangeal (MCP) joint. Generally, the cords or contractures are painless, but, rarely, tenosynovitis can occur and produce pain. The ulnar side of the hands is affected most often, with the fourth and fifth fingers affected earliest. Generally, the thumb and the index finger are spared. The disease begins in the palm and moves proximal to distal, with the metacarpophalangeal (MCP) joints affected before the proximal interphalangeal (PIP) joints.
In Dupuytren's contracture, the palmar fascia within the hand becomes abnormally thick, which can cause the fingers to curl and can impair finger function. The main function of the palmar fascia is to increase grip strength; thus, over time, Dupuytren's contracture decreases patients' ability to hold objects. Patients may rarely report pain, aching and itching with the contractions.
People with severe involvement often show lumps on the back of their finger joints (called “Garrod pads”, “knuckle pads”, or “dorsal Dupuytren nodules”) and lumps in the arch of the feet (plantar fibromatosis). In severe cases, the area where the palm meets the wrist may develop lumps. Severe Dupuytren disease may also be associated with frozen shoulder (adhesive capsulitis of shoulder), Peyronie disease of the penis, increased risk of several types of cancer, and risk of early death, but more research is needed to clarify these relationships.
Top of page
Related conditions
- Peyronie's disease - curvature of the penis
- Plantar fibromatosis - callus under the foot and possible curling under of toes
- Garrod's pad - pads on the back of knuckles of fingers
- Frozen shoulder - shoulder that develops stiffness and limited range of motion
Top of page
Causes
Suspected, but unproven, causes of Dupuytren's contracture include trauma, diabetes, epilepsy and therapy with phenytoin. No proven evidence links hand injuries or specific occupational exposures to a higher risk of developing Dupuytren’s. Some speculation links the condition or its onset may be triggered by, physical trauma such as sustained manual labor or over-exertion of the hands. However, the fact that Dupuytren's is not connected with handedness undermines this claim.
Risk factors
Dupuytren's contracture is a non-specific affliction, but primarily affects:
- People of Scandinavian or Northern European ancestry; it has been called the "Viking disease", though it is also widespread in some Mediterranean countries (e.g., Spain and Bosnia);
- Men rather than women (men are ten times as likely to develop the condition);
- People over the age of 40;
- People with a family history (60% to 70% of those afflicted have a genetic predisposition to Dupuytren's contracture);
- Rock climbers
- Alcoholics
Top of page
Pathophysiology
The palmar fascia becomes abnormally thick due from a change of collagen type. Normally, the palmar fascia consists of collagen type I, but in Dupuytren sufferers, the collagen changes to collagen type III, which is significantly thicker than collagen type I. The contracture sets in slowly.
Top of page
Diagnosis
Treatment is indicated when the so-called table top test is positive. With this test, the patient places his hand on a table. If the hand lies completely flat on the table, the test is considered negative. If the hand cannot be placed completely flat on the table, leaving a space between the table and a part of the hand as big as the diameter of a ballpoint pen, the test is considered positive and surgery or other treatment may be indicated. Additionally, finger joints may become fixed and rigid. Treatment using radiation therapy begins at an earlier stage. Radiation therapy is most effective when nodules and cords first appear, and before contracture begins.
The initial description of Dupuytren’s disease diathesis included 4 factors:
- the patient is below the age of 50 years
- positive family history
- both of the hands are affected
- ectopic lesions (Peyronie's disease, Knuckle pads and Ledderhose disease).
Hindocha et al. reevaluated these 4 factors and modified them. Two additional factors were added: male gender and age at onset below 50 years.
Top of page
Treatment
Treatment involves one or more different types of treatment with some hands needing repeated treatment.
The main categories listed by the International Dupuytren Society in order of stage of disease are Radiation Therapy, Needle Aponeurotomy (NA), Collagenase Injection (Xiaflex) and Hand Surgery.
Radiation Therapy is effective at the early nodules and cords stage "Stage N" and also used at the N/I stage of 10 degrees or less of deformation.
Needle Aponeurotomy is most effective at "Stage I" of 6-45 degrees of deformation. It is also used at less than 6 degrees and more than 45 degrees of deformation.
Collagenase Injection (Xiaflex) is most effective at "Stage I". It is also used at "Stage II" of 46-90 degrees of deformation.
Hand Surgery is effective at Stages I- Stage IV.
Early Stage: Radiation Therapy
Dupuytren’s may be treated at the nodules, cords and early finger deformation stages with Radiation therapy. In Germany and parts of the U.S., radiotherapy is one of the main treatments.
X-Ray and more recently E-beam radiation are used.
The purpose of radiotherapy is to stop disease progression. It has a documented success rate of 85%.
Surgical
Limited Fasciectomy

Limited/selective fasciectomy removes the pathological tissue, and is a common approach.
During the procedure:
- The patient is under regional or general anesthesia.
- A surgical tourniquet prevents blood flow to the limb.
- The skin is often opened with a zig-zag incision but straight incisions with or without Z-plasty are also described- and may reduce damage to neurovascular bundles.
- All diseased cords and fascia are excised. The excision has to be very precise to spare the neurovascular bundles. Because not all the diseased tissue is visible macroscopically, complete excision is uncertain.
- After the tissue is removed, the surgeon closes the incision.
- In the case of a shortage of skin, the transverse part of the Zig-Zag incision is left open.
- Stitches are removed 10 days after surgery.
After surgery the hand is wrapped in a light compressive bandage for one week. Patients start bending and extending their fingers as soon as the anesthesia has resolved. Hand therapy is often recommended. Approximately 6 weeks after surgery patients are able to completely use their hand.
The average recurrence rate is 39% after a fasciectomy after a median interval of about 4 years.
Dermofasciectomy
Dermofasciectomy is a surgical procedure that is mainly used in recurrences and for patients with a high chance of recurrence. Just like the limited fasciectomy, the dermofasciectomy excises diseased cords, fascia and the overlying skin. The skin is then closed with a skin graft, usually full-thickness, consisting of the epidermis and the entire dermis. In most cases the graft is taken from the elbow flexion crease or the proximal inner side of the arm. This place is chosen, because the skin color best matches the palm's skin color. The skin on the proximal inner side of the arm is thin and has enough skin to supply a full-thickness graft. The donor site can be closed with a direct suture.
The graft is sutured to the skin surrounding the wound. For one week the hand is protected with a dressing. The hand and arm are elevated with a sling. The dressing is then removed and careful mobilization can be started, gradually increasing in intensity. After this procedure the recurrence of the disease can be low but the re-operation and complication rate may be high.
Free Vascular Flap
In severe cases a free vascular flap may be preferred and is thought to reduce recurrence. A one-year follow-up of a single patient was described. This patient had not experienced recurrence.
Top of page
Minimally-Invasive Surgery
Segmental Fasciectomy with/without Cellulose
Segmental fasciectomy involves excising part(s) of the contracted cord so that it disappears or no longer contracts the finger. It is less invasive than the limited fasciectomy, because not all the diseased tissue is excised and the skin incisions are smaller.
The patient is placed under regional anasthesia and a surgical tourniquet is used. The skin is opened with small curved incisions over the diseased tissue. If necessary, incisions are made in the fingers. Pieces of cord and fascia of approximately one centimeter are excised. The cords are placed under maximum tension while they are cut. A scalpel is used to separate the tissues. The surgeon keeps removing small parts until the finger can fully extend.Patients start with active mobilization the day after surgery. They wear an extension splint for two to three weeks, except during physical therapy.
The same procedure is used in the segmental fasciectomy with cellulose implant. After the excision and a careful haemostasis, the cellulose implant is placed in a single layer in between the remaining parts of the cord.
After surgery patients wear a light pressure dressing for four days, followed by an extension splint. The splint is worn continuously during nighttime for eight weeks. During the first weeks after surgery the splint may be worn during daytime.
Percutaneous Needle Fasciotomy
Needle aponeurotomy is a minimally-invasive technique where the cords are weakened through the insertion and manipulation of a small needle. The cord is sectioned at as many levels as possible in the palm and fingers, depending on the location and extent of the disease, using a 25 Gauge needle mounted on a 10 ml syringe. Once weakened, the offending cords can be snapped by putting tension on the finger(s) and pulling the finger(s) straight. After the treatment a small dressing is applied for 24 hours. After these 24 hours patient are able to use their hands normally. No splints or physiotherapy are given.
The advantage of needle aponeurotomy is the minimal intervention without incision (done in the office under local anesthesia) and the very rapid return to normal activities without need for rehabilitation, but the nodules may resume growing.
Extensive Percutaneous Aponeurotomy and Lipografting
A technique introduced in 2011 is extensive percutaneous aponeurotomy with lipografting. This procedure also uses a needle to cut the cords. The difference with the percutaneous needle fasciotomy is, that the cord is cut at many places. The cord is also separated from the skin to make place for the lipograft that is taken from the abdomen oripsilateral flank. This technique shortens the recovery time. The fat graft results in supple skin.
Before the aponeurotomy, a liposuction is done to the abdomen and ipsilateral flank to collect the lipograft. The treatment can be performed under regional or general anesthesia. The digits are placed under maximal extension tension using a firm lead hand retractor. The surgeon makes multiple palmar puncture wounds with small nicks. The tension on the cords is crucial, because tight constricting bands are most susceptible to be cut and torn by the small nicks, whereas the relatively loose neurovascular structures are spared. After the cord is completely cut and separated from the skin the lipograft is injected under the skin. A total of about 5 to 10 ml is injected per ray.
After the treatment the patient wears an extension splint for 5 to 7 days. Thereafter the patient returns to normal activities and is advised to use a night splint for up to 20 weeks.
Top of page
Non-Surgical
Collagenase

Clostridial collagenase is a pharmaceutical treatment option. The cords are weakened through the injection of small amounts of the enzyme collagenase, which breaks peptide bonds in collagen.
The treatment with collagenase is different for the MCP joint and the PIP joint. In a MCP joint contracture the needle must be placed at the point of maximum bowstringing of the palpable cord. The treatment consists of one injection with 0.58 mg 0.25 ml. collagenase clostridium histolyticum (CCH).
The needle is placed vertically on the bowstring. The collagenase is distributed across three injection points. For the PIP joint the needle must be placed not more than 4 mm distal to palmar digital crease at 2–3 mm depth. The injection for PIP consists of one injection filled with 0.58 mg CCH 0.20 ml. The needle must be placed horizontal to the cord and also uses a 3-point distribution. After the injection the patient’s hand is wrapped in bulky gauze dressing and must be elevated for the rest of the day. After 24 hours the patient returns for passive digital extension to rupture the cord. Moderate pressure for 10–20 seconds ruptures the cord.
After the treatment with collagenase the patient should use a night splint and perform digital flexion/extension exercises several times per day for 4 months.
Top of page
Prognosis
Dupuytren’s disease has a high recurrence rate, especially when a patient has so called Dupuytren’s diathesis. The term diathesis relates to certain features of Dupuytren's disease and indicates an aggressive course of disease.
Minimally invasive therapies may precede higher recurrence rates. Recurrence lacks a consensus definition. Furthermore, different standards and measurements follow from the various definitions.
Top of page
Postoperative care
Postoperative care involves hand therapy and splinting. Hand therapy is prescribed to optimize post-surgical function and to prevent joint stiffness.
Besides hand therapy, many surgeons advise the use of static or dynamic splints after surgery to maintain finger mobility. The splint is used to provide prolonged stretch to the healing tissues and prevent flexion contractures. Although splinting is a widely used post-operative intervention, evidence of its effectiveness is limited, leading to variation in splinting approaches. Most surgeons use clinical experience to decide whether to splint. Cited advantages include maintenance of finger extension and prevention of new flexion contractures. Cited disadvantages include joint stiffness, prolonged pain, discomfort, subsequently reduced function and edema.
A third approach emphasizes early self-exercise and stretching.


